Research

Projects
Small molecule inhibitor of two component histidine sensor kinase against multi drug resistance Staphylococcus aureus: A novel anti-virulent strategy
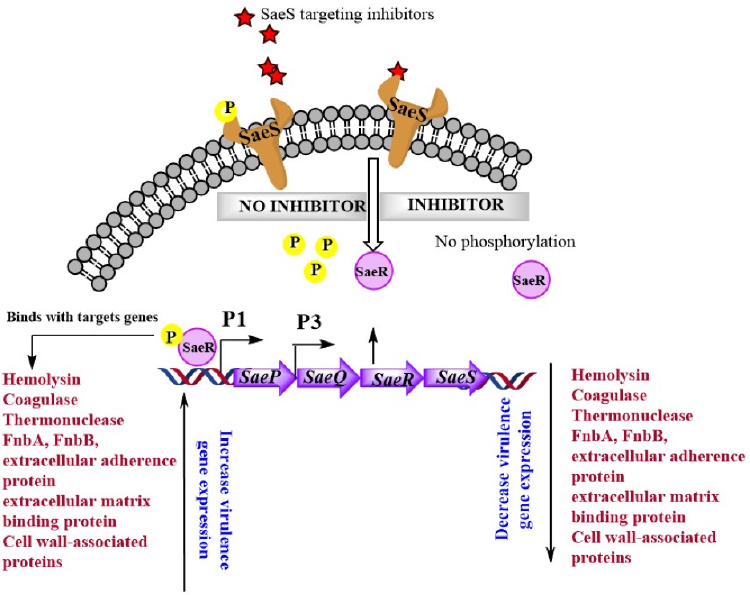
Methicillin-resistant Staphylococcus aureus (MRSA) is the most common cause of hospital-acquired infection, which exhibits as surgical site infections, bacteremia, and sepsis. The frequent exposure of S. aureus to various antimicrobials due to their higher usage has driven the emergence of new clones that are resistant to multiple antibacterial drugs. The rapid evolution of multi-drug resistant S. aureus strains along with higher morbidity, graft rejection and healthcare related infections has increased the complexity of pathogenesis. Among the major virulence regulators of S. aureus, the sae locus has been studied extensively due to their significant role in establishment of staphylococcal infections but, to our knowledge, the locus has not been utilized to develop or screen virulence inhibitors. Targeting such two component sensor kinase is a novel approach to identified antivirulent candidates without compromising the cell growth. Currently we are working on high through put screening of various small molecule libraries including FDA approved drugs, flavonoids, natural compounds and kinase inhibitors. We have identified Avirasa as a potent inhibitor which selectively inhibits phosphorylation of saeS and hence attenuated the virulence gene expression. Furthermore, the “combinatorial therapy” could be applied for treatment of staphylococcal infections by using the synergistic effect between novel small molecule inhibitors that could inhibit virulence gene expression along with bacterial resistance mechanism and sub-therapeutic dose of antimicrobial drugs.
Nanobiorobotics-based antibacterial approach for targeted eradication of multiple drug resistant bacteria
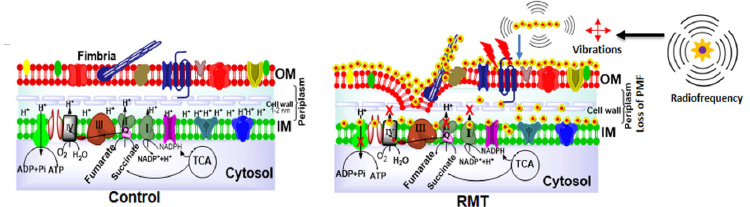
Emergence of antibiotic resistant strains worldwide demanded provision of novel therapeutic agents, e.g. small peptides, antivirulent drugs and nanoconstructs. Massive research has been conducted on antibacterial property of nanoparticles in relation to their resistance free nature and high pervasiveness due to nano scale size. Various fabrication strategies are now being executed to make nano conjugates e.g. nucleotides and antibodies complexed with nanoparticles. Comparatively we are proposing theranostic approach to capture resistant bacterial strains by biocompatible nanobiorobots and their targeted killing due to loss of bacterial membrane integrity by exposure of radiofrequency. This approach resolves the requirement of large sample volume, low shelf life (rapid degradation) and toxicity issues. Our research dimension is focused further to exploit the structural moieties ofpathogens which can be a novel target for newly developed nanomedicines based on host innate and adaptive immune system.
Antivirulence approach for dealing antimicrobial resistance in vibriosis
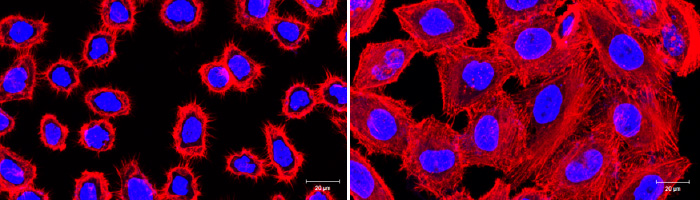
LEFT: HeLa cells infected with V. vulnificus detaches, lose shape due to disruption of cytoskeleton (red) and eventually dies. RIGHT: HeLa cells were protected from the cytoskeletal disruption and cell death by antivirulent small molecule treatment of V. vulnificus targeting a master virulence transcriptional regulator in the pathogen. Scale bar: 20 µm.
Vibriosis is an under-reported and under-recognized human illness caused by the species of family Vibrionaceae (excluding toxigenic Vibrio cholerae O1 and O139, which cause cholera), most commonly Vibrio vulnificus and V. parahaemolyticus. These pathogens typically cause gastrointestinal infections and may cause bacteremia, necrotizing wound infections or extra-intestinal infections with a high rate of mortality especially in immunocompromised individuals. Recently a high rate of antimicrobial resistance (AMR) has been developed and reported in these pathogens. AMR develops when a microbe adapts to the continued and/or irrelevant use of antibiotics/antimicrobials and eventually completely escapes and become resistant to the antimicrobial agent. AMR has emerged over decades and is endangering the development in human health sector. The lack of new antibiotics and emergence of AMR urges the need of an alternative solution. Our lab is working in this direction by employing antivirulent therapeutic approach via development of diverse orthogonal/non-orthogonal reporter platforms for identification of small molecules targeting master virulence regulators to subjugate the pathogenicity of pathogen. A major advantage of antivirulence approach is that the normal bacterial growth remains unaffected and thereby it reduces the selection pressure for resistance development in the microbe.
Mechanochemical antibacterial approach to sensitize and eradicate multiple drug resistant bacteria
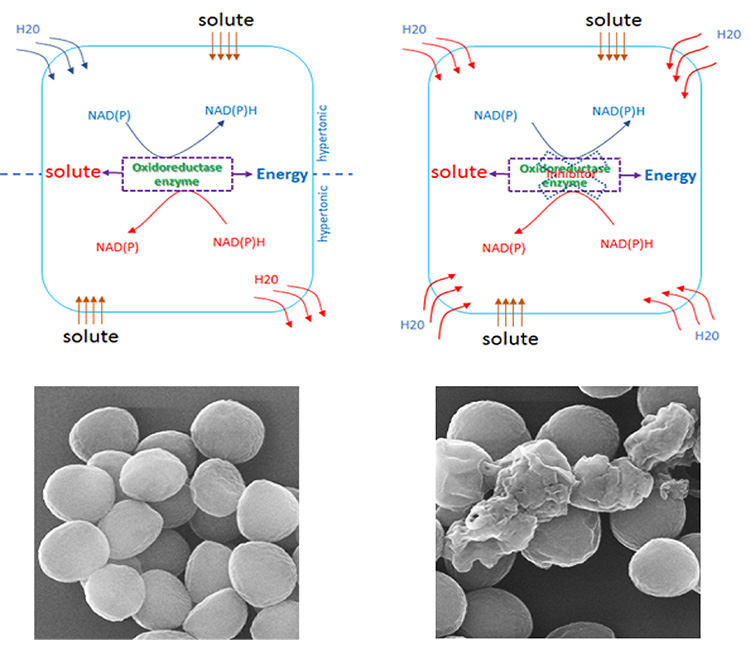
Bacteria use their compatible solute metabolism system to deal with changing of environmental condition to maintain their shapes and sizes. When bacteria are exposed to hypotonic or hypertonic condition leading to intracellular water decreasing or vice versa, the bacteria are undergoing osmotic pressure. To prevent cell shrinking/swelling, the solute uptake or biosynthesis and metabolism would be induced to keep the water level balance. These systems are regulated by a membrane associated solute uptake system and an NAD(P)/NAD(P)H dependent oxidoreductase enzyme. In most of cases, these compatible solutes are favor to be uptake if present in the environment whereas their intracellular levels are control by the enzyme. As the result, depending on certain condition, the regulation of protein expression or activity of the enzyme will determine the solute level inside the cell. By understanding this mechanism, we are targeting compatible solute metabolism system to develop the mechanochemical method for drug discovery to treat certain pathogenic bacteria.
